Ebola and innovation: examining the approach to the Nord Kivu epidemic
Natalie Roberts
This article was first published in March 2020 for the Humanitarian Practice Network.
Within four months of the first notification of Ebola cases in August 2018, the Nord Kivu (and Ituri) Ebola epidemic had become the second-largest on record. Notwithstanding a rapid and massive mobilisation of resources, the outbreak continued beyond the most pessimistic predictions and the case fatality rate (the proportion of people with the infection who die from it) remained static at 66%. Despite numerous lesson-learning exercises following the Ebola epidemic in West Africa in 2014–2016, and despite the development of new vaccines and treatments, after 3,444 cases and 2,264 deaths it is difficult to claim that outcomes are better this time around.
At the same time, the response to the Nord Kivu epidemic was marked by extensive innovation and the rapid translation of ideas and inventions into practice. While previous outbreaks of Ebola had been small and arguably self-limiting, the duration and scale of the epidemic in West Africa led to the development of ideas, techniques and products which were finally put to the test in the DRC. Some are already proving successful, notably the use of a vaccine which seems to have not only protected many health workers from infection, but also had an impact on the scale and duration of the epidemic.
There are three key questions in the design of any epidemic response: how to protect the responders; how to reduce the number of infected people, i.e. the incidence of the disease; and how to reduce the number of dead, i.e. the lethality of the disease. These questions are framed within the timescale and geographical spread of an epidemic, the social and political dimensions of the response and the activities of the institutions in charge of it. The aim should be to minimise the negative political and socio-economic consequences of the epidemic, or at least not exacerbate them.
In the aftermath of the West African epidemic, MSF CRASH and Epicentre, an MSF satellite institution in charge of epidemiology, began a research study to investigate different practices proposed or tested by responders to address these questions. Following the Nord Kivu outbreak this work will be reviewed and updated, to inform future approaches to Ebola and epidemics in general. Some initial considerations are detailed here.
Protecting responders
In an outbreak of infectious disease the initial priority is to protect the responders, who are essential to the success of the intervention. This is imperative in an Ebola epidemic, as health workers are at disproportionate risk of infection. Many fall sick or die from the disease, while fear of infection leads others to stop working and health facilities to close. This reduces the capacity of the response and weakens general healthcare provision. In addition, health workers who fall sick with Ebola increase the disease burden and therefore the workload for those still working. Infected health workers also act as spreaders of the disease, and in previous epidemics have been identified as one of the principal mechanisms of transmission.
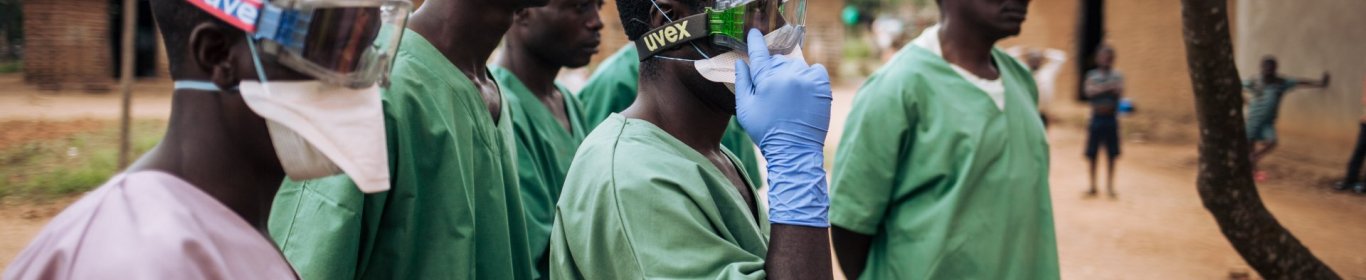
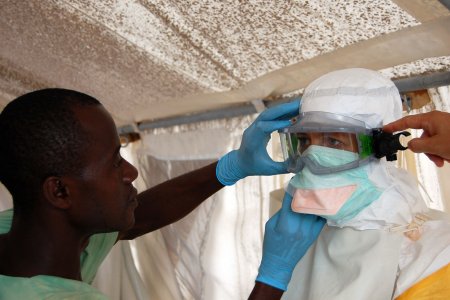
Organisations responding to Ebola outbreaks have adopted a model of Personal Protective Equipment (PPE) intended to decrease the risks to health workers and other frontline responders likely to come into contact with infected bodily fluids. However, the typical configuration of ‘Full PPE’ is hot, heavy, expensive, restrictive and not adapted to the situations in which it is used. Medical personnel working in treatment centres complain that patient care becomes impractical or impossible. It is unrealistic for personnel in peripheral health facilities to continuously wear ‘Full PPE’ just in case a patient with non specific symptoms turns out to be suffering from Ebola. Burial teams complain that the yellow suits and facemasks provoke fear and aggression, resulting in physical attacks. Attempting to address these problems, MSF France trialled algorithms in Nord Kivu health facilities to recommend the appropriate configuration of PPE according to the probable risk each patient poses to the health worker. ALIMA, a French, African-based NGO, developed the CUBE, a transparent biosecure unit designed to facilitate the monitoring and care of Ebola patients while reducing time health workers spend in PPE.
Meanwhile, a vaccine shown to confer protection against infection from ten days after administration was supposed to be available to all ‘frontline’ Ebola workers in DRC. Despite initial concerns, acceptance was high and there was consistent demand for vaccination. However, a number of challenges and shortcomings limited the full impact of the intervention. First, traditional healers and staff in private clinics provide a considerable proportion of health services in DRC and are at high risk of infection, but were not included in the eligibility criteria. Second, although few eligible workers refused, not all eventually received a vaccine. Waiting lists were long and the vaccination process, under the management of WHO and the Congolese Ministry of Health (MOH), was complex and slow, partly as a result of uncertainty about the use and study of an unregistered product. Some MSF and MOH staff working in MSF facilities believed that they were not allowed time away from work; side-effects of vaccination can be debilitating and last several days. Some reported that high demand for limited stocks had made the vaccine a commodity, and suspected that their dose had been sold to someone else. Despite these shortcomings, however, the use of the vaccine during the Nord Kivu epidemic is likely to be the primary reason for the significant reduction in health worker infections compared to West Africa.
In addition to preventive vaccination, WHO recommends antibody therapies be considered as prophylaxis for ‘frontline’ health workers with a high risk of exposure to Ebola. However, Congolese health workers, particularly if not working within dedicated Ebola facilities, were not generally aware of this possibility. Incidents of exposure were under-reported, and post-exposure prophylaxis was under-used.
Reducing the number of affected people
As Ebola sufferers are thought to become contagious only when symptomatic, efforts have long been made to convince people to submit to isolation as soon as they fall ill, to try to curtail disease spread. These practices often fail. Who would agree to be admitted to an Ebola centre knowing that the majority of patients do not leave alive? Who would go to an Ebola centre just to be tested, when they are more likely to be suffering from malaria or gastroenteritis?
One common approach to try to stop transmission is contact tracing, where people who have come into contact with a person confirmed as infected are identified and followed up daily for 21 days, with the aim of isolating them rapidly if they become ill. Congolese contact tracing teams struggled due to the scale and geographic spread of the epidemic, the mobility of the local population and the reluctance of symptomatic contacts to be placed in isolation. The World Food Programme began providing food to contacts so they wouldn’t have to leave their homes to go to the market and could be more easily monitored. This probably increased compliance with monitoring, but it also increased the number of people identifying themselves as a contact, many unnecessarily. As well as further overwhelming the system, this could help explain why men were disproportionately listed as contacts, although women and children were actually more likely to be infected.
Understanding the mechanisms of Ebola transmission is essential to designing a response that aims to reduce the number of infected individuals. In DRC, people were most frequently infected through contact with a sick member of their family or social group, often while providing care. Nosocomial transmission was also important, as vaccinating health workers did not prevent transmission from patient to patient linked to poor hygiene practices within health facilities, such as the sharing of beds or the reuse of medical equipment. The third major risk factor for infection in DRC was participation in funeral rites.
Disease control practices that rely on behaviour change are slow and can be resisted. For example, it is unrealistic to expect family members to stop caring for sick loved ones, the main risk factor behind community transmission. The sick in Nord Kivu are usually cared for at home, with visits to local traditional healers, pharmacies or clinics. Government health facilities are considered mainly for illnesses that are not resolving, with relatives staying to provide general nursing care. The practice of separating sick patients from their family to isolate them in an Ebola centre is considered unacceptable, and centres are unwelcoming and provide little support for families. Caregivers have few options but to go home and wait to see if they themselves fall sick.
Rather than attempting to change long-standing behaviour, MSF France and Epicentre wanted to use antibody therapies as postexposure prophylaxis (PEP) for family caregivers of confirmed Ebola cases. This would offer individuals at elevated risk of having been infected a potentially effective intervention to stop them developing the disease, and to encourage them to comply with monitoring. If PEP is completely effective the caregiver would not become sick, so should not become contagious. Even if not, the disease would potentially be milder and they could be isolated and given appropriate care as soon as symptoms appeared. However, this practice was not sanctioned by the WHO and Congolese health authorities, apparently because of concerns about supply or that antibody treatments might reduce the long-term protective effect of the vaccine.
Various practices attempted to reduce nosocomial transmission. Decontamination of health facilities reporting an Ebola case became a routine component of the response, but is labourintensive and does not prevent recontamination of that facility if another infected patient arrives. Decontamination teams wearing suits and masks also signal the presence of Ebola, deterring patients from using centres. Various actors distributed additional hygiene supplies and provided staff training, but the large number of health facilities and the fact that most were understaffed and lacked basic infrastructure such as water supply made this activity relatively useless.
Ultimately, vaccination could prove an effective method to stop spread, or at least to prevent large outbreaks. Two vaccines were made available for study during the DRC epidemic, including one whose efficacy had been proven in West Africa. The challenge remains to design strategies that identify the right people to receive the right vaccine at the right time, taking into consideration supply limitations and constraints such as ultralow temperature cold chain management. Difficulties in contact tracing meant that the WHO-led ring vaccination strategy, which depends on an accurate and comprehensive identification of contacts, did not adequately control the spread of disease.
Reducing the number of deaths
A clinical trial of experimental treatments during the Nord Kivu epidemic identified two effective monoclonal antibody treatments effective in the treatment of Ebola.
For an increased chance of survival, Ebola sufferers should receive one of these curative drugs together with supportive care, such as fluid rehydration, adapted to the severity of illness. Neither of these measures is effective when the illness is too advanced, so early detection and treatment is crucial.
Medical responders, notably ALIMA, WHO and the MOH, have pushed the boundaries of patient care. Innovations such as the CUBE and staff vaccination, the deployment of intensive care physicians and the use of critical care models have allowed high-level supportive care to be adapted to individual patient circumstances in a high-risk environment.
The availability of new treatments and laboratory diagnostics in DRC was suggested to encourage sick people to present earlier for testing and treatment. However, Congolese health promoters and local media spreading the message about treatments did not obviously reduce the average time between the development of symptoms and admission to an Ebola centre. Apart from centres being inhospitable and frightening, admission required passing via a gatekeeper, oꢀen a local healthcare provider. In response, MSF created small isolation areas within existing local health facilities where symptomatic patients could receive care while being tested for Ebola, reassured that they would be referred to a dedicated Ebola centre only if test results were positive. In Beni this appears to have reduced the average delay in treating patients who present to these health facilities. However, demonstrating an impact on overall case fatality rates would require the model to be deployed and studied more widely. Some in MSF recommended financial assistance for Ebola patients or their families, to reduce the social and economic impact of infection with a stigmatising disease that results in prolonged disability or death. Apart from ensuring that affected individuals benefit directly from the massive resources dedicated to the Nord Kivu and Ituri Ebola response, this was surmised to be the quickest way to encourage people to positively engage with actors involved in the response. However, others within the organisation considered this too sensitive or complex, and the idea was not developed further. Direct financial support to Ebola victims was ultimately not adopted by any actor involved in the response.
Controversies: science versus innovation; science versus ethics
It is difficult to assess new practices ‘scientifically’, as they areby necessity guided by operational reasoning and experience, rather than pre-existing evidence. Ebola outbreaks have become notable for the number and range of responders, all acting under time pressure to try to contain the epidemic. This leads to a maelstrom of promising ideas being implemented all at once: the opposite of traditional scientific research, where only one variable at a time is adjusted and the impact evaluated. The interactions between multiple interventions can result in outcomes greater than the sum of their parts, but are difficult to decipher: if a combination of early diagnosis and treatment and care provides the highest likelihood of survival for Ebola victims, where best to expend energy to reduce the overall case fatality rate? Traditional research is slow but thorough; innovative empirical practice has the benefit of speed. In any practice there is an obligation to document, analyse and evaluate in an attempt to improve outcomes. However, formal research protocols, designed to generate statistically significant evidence rather than inform current practice, must be carefully considered to avoid unnecessarily slowing the implementation of innovative ideas.
Attempting to modify practice in a timely but ethical manner within a context of uncertainty is challenging for humanitarian practitioners. As in West Africa, confusion and tensions arose around the use of ‘experimental’ or unregistered products during the Nord Kivu epidemic. For example, despite participating in frontline worker vaccination activities, MSF did not initially recommend vaccination to its own staff or stop unvaccinated personnel from entering high-risk situations. Internal debate focused on the ethics of endorsing an unregistered product and on giving personnel the right to choose without influencing their decision. The information provided to staff was ambiguous, often reflecting the perspective of an individual manager or MSF section. Some MSF managers concluded that there must be hidden concerns about vaccine safety given it was still part of a clinical study (which was in fact to confirm effectiveness). Others worried that vaccinated staff might develop a false sense of security and take excessive risks, so wanted to recommend only (unproven) PPE measures. One MSF section claimed it irresponsible to allow staff to expose themselves to risk by being in contact with Ebola patients without having been vaccinated; others felt that banning unvaccinated staff from treatment centres would put undue pressure on them to accept the vaccine. This issue was only resolved months after the start of the outbreak, when it became obvious that vaccinated staff were rarely becoming infected.
What next for Ebola and other epidemic responses?
With the Nord Kivu epidemic slowing, there is an opportunity for all actors to reflect on successes and failures in the response. For MSF, dissecting and understanding what approaches were attempted or rejected by different responders at different stages of the epidemic could help in creating working hypotheses for the future. Analysis of the response will lead to considerations specific to Ebola, but also reinforce recommendations for planning any epidemic response, for example regular meetings to clarify response intentions with international and national actors and the health authorities in countries where outbreaks are most likely to occur. Given the likelihood of similar ‘Public Health Emergencies of International Concern’, it is important for MSF to study the organisation of the Ebola response, its management and funding, the actors involved and MSF’s own complicated relationship to it. Finally, examining the process of how humanitarian actors learn via trial and error during scientific, ethical and political uncertainty can contribute to the development of a more robust operational response to dangerous epidemics.
To cite this content :
Natalie Roberts, “Ebola and innovation: examining the approach to the Nord Kivu epidemic”, 25 mars 2021, URL : https://msf-crash.org/en/blog/humanitarian-actors-and-practices/ebola-and-innovation-examining-approach-nord-kivu-epidemic
If you want to criticize or develop this content, you can find us on twitter or directly on our site.
Contribute



Add new comment